 Evaluation of Antidiabetic effects of Ficuts Racemosa Evaluation of Antidiabetic effects of Ficuts Racemosa
Extracts in Diabetic induced Rats - by Anup Kumar Maurya1*,
Smriti Tripathi 1, Monica Kahrana2, Zabeer Ahmed3, Ram
Kumar Sahu4
a) Abstract b) Introduction
c) Materials and Methods
d) Results e) Discussion |
e) References |
 |
|
The aim of this study was to investigate the antidiabetic effect of methanol extract of
leaves of Ficus racemosa. This was tested in normal and Streptozotocin (STZ)-induced
diabetic rats, using oral administration of 50% and 70% methanol extract (250 and 500
mg/kg body weight) of Ficus racemosa leaves. After the oral administration of 50%
methanol and 70% methanol extracts at doses of 250 and 500 mg/kg body weight, blood
glucose levels were monitored at specific intervals and it was found that they were
significant lowered. Glibenclamide was used as a standard drug at a dose of 0.25 mg/kg.
The effect of the extracts on diabetes induced hyperlipidemia was analyzed where the
extracts significantly lowered the elevated total cholesterol, triglycerides (TGL) and low
density lipoprotein (LDL) level while increased the High density lipoprotein (HDL).The
experimental data revealed that both extracts has significant antdiabetic activity in
Streptozotocin-induced rats compared to the standard drug.
Keywords: Ficus racemosa, antidiabetic, glibenclamide, streptozotocin
|
Back to Top  |
|
 |
 |
|
Diabetes mellitus is an endocrine disorder, which characterized with hyperglycemia.
Diabetes mellitus has affected a considerable population and in the future it will be a
major disorder affecting people across the globe, irrespective of sex, age and ocioeconomic
status. In modern medicine, no satisfactory effective therapy is still available to cure diabetes mellitus. Insulin has proved to be effective to some extent in increasing the life expectancy of diabetic patients, but is not a permanent solution since there are many drawbacks of this therapy. But it can be managed by exercise, diet and chemotherapy[1-3]. Hence the use of ethnobotanicals has a long folkloric history for the treatment of blood sugar abnormalities and it is devoid of adverse effect. The World Health Organisation has estimated that 80% of the world’s population use botanical medicine fortheir primary health care needs[4].
Ficusracemosa(syn. FicusglomerataRoxb.) is a species of plant in the Moraceae family. Popularly known as the cluster tree or goolar (gular), this is native to Australia, SouthEast Asia and the Indian Subcontinent. It is found throughout the year, grows in evergreen forests, moist localities and bank of streams, deciduous forests, often cultivated in villages for shade and its edible fruits. The tree is up to 18 m high, leaves ovate, ovatelanceolate or elliptic, sub acute, entire and petiolate. Figs subglobose or pyriform, red when ripe, borne in large clusters, on short, leafless branches emerging from the trunk and the main branches. Among the natives it is known for its tasty fruits. The children consume fruit of Doomar with taste. Doomar fruits are also used as medicine. The roots are used as a medicine against hydrophobia. Its fruits are effective against leprosy, diseases of the blood, fatigue, bleeding nose and cough. Its bark is helpful against asthma and its leaves are used against bronchitis. It is used as carminative, astringent, vermifuge and an antidysentery drug[57]. The extract of fruit is used in diabetes and leucoderma. The plant is used locally to relieve inflammation of skin wounds, lymphadenitis, sprains and fibrositis. The alcoholic extract of the stem bark of the plant possessed antiprotozoal activity against Entamoeba histolytica. It is used in the treatment of mumps, smallpox, heamaturia and inflammatory conditions. The bark and leaves are useful in treating diabetes[8]. Though there is no scientific evidence to support the antidiabetic effect of Ficusracemosa, tribal men continue to use the plant in the management of diabetes. The aim of this investigation was to ascertain the scientific basis for the use of this plant in the management of diabetes, using streptozotocin induced diabetic rats. |
Back to Top  |
|
 |
 |
|
|
Plant materials: The leaves part of Ficusrecemosawere collected from the forest area of IIM garden, Jammu and Kashmir, India, during the months of October 2010. The species was identified by the local people during the time of collection and later on authentication was made by botanist Dr. S. N. Sharma Department of Taxonomy, I.I.I.M, Jammu, India and a voucher specimen was deposited in the Herbarium of Department of Botany, IIIM Jammu. The leaves were shade dried, reduced to coarse powder and stored in airtight container till further use.
Preparation of extract: 1 Kilogram of leaves powdered drug were packed in soxhlet apparatus and extracted with 50% methanol and 70% methanol separately. The solvents were removed by distillation from both extracts and the last traces of solvent being removed under reduced pressure. Suspension of 50% methanol extract and 70% methanol extract were prepared using 1% Tween 80 separately and both suspensions were divided in two doses 250 mg/kg and 500 mg/kg body weight. The doses of 250 mg/kg and 500 mg/kg body weight of 50% methanol extract were abbreviated as AKS001 and AKS002 respectively. The doses of 250 mg/kg and 500 mg/kg body weight of 70% methanol extract were abbreviated as AS001 and AS002 respectively.
Experimental animals: Male and female wistar albino rats having weight 180230 gm bred in the Animal House, Institute of Integrative Medicine (IIIM), Formerly Regional Research Laboratory (CSIR), Jammu, were used. The animals were housed were kept in quarantine for 10 days under standard husbandry conditions. All animal used in experiments were approved by the Institutional Animal Ethic Committee (IAEC), of Indian Institute of Integrative Medicine, IIIM (Formerly Regional Research Laboratory) (CSIR) Jammu. 3oC, Relative humidity 65 ±10%) for 12 hrs in dark and light cycle respectively and were given standard food and water ad.libitum.
Acute oral toxicity study: Acute oral toxicity was performed by following OECD guideline – 420 fixed dose procedure for methanol extract and it was found that dose increasing up to 2000 mg/kg body wt. shown no toxicity or mortality in experimental rats. The LD50 of the ethanol and aqueous extract as per OECD guidelines – 420 is greater then 2000 mg/kg[9,10].
Oral glucose tolerance test (OGTT): The oral glucose tolerance test was performed in overnight fasted (18 hours) normal rats. The rats were divided into seven groups (n= 6) and were administered drinking water. Group I served as normal control rats administered drinking water; Group II glucose control rats administered; Group III rats administered standard drug glibenclamide (0.25 mg/kg); Group IV diabetic rats administered AKS001; Group V diabetic rats administered AKS002; Group VI diabetic rats administered AS001; Group VII diabetic rats administered AS002. Glucose (1.5; 10% sol. g/kg) was fed 30 minutes prior to the administration of the extracts. Blood was withdrawn from the retroorbital sinus after 0, 30, and 90 minutes of extract administration, and the plasma obtained after centrifugation at 3000 rpm was estimated for fasting plasma glucose levels using a glucose oxidase–peroxidase glucose estimation kit[11].
Induction of noninsulin dependent diabetes mellitus (IDDM): Diabetes mellitus was induced by single intraperitoneal injection of freshly prepared Streptozotocin (STZ 45 mg/kg body weight) in 0.1M citrate buffer (pH−4.5) in a volume of 1ml/kg body weight. Diabetes was established in these STZ treated rats over a period of 7 days. The control animals were treated with citrate buffer (pH−4.5). After 7 days the blood was collected by retroorbital and the plasma glucose level of each rat was determined.[12,13].
Experimental design: The animals were segregated into seven groups of six rats each. The extract was administered for 28 days. Group I served as normal control rats administered drinking water daily for 28 days; Group II diabetic control rats administered drinking water daily for 28 days; Group III diabetic rats administered standard drug glibenclamide (0.25 mg/kg); Group IV diabetic rats administered AKS001; Group V diabetic rats administered AKS002; Group VI diabetic rats administered AS001; Group VII diabetic rats administered AS002 for 28 days.
The fasting glucose levels were determined on days 0, 7, 14 and 28 of extract administration. During the experimental period, the rats were weighed daily and the mean change in body weight was calculated.
Estimation of biochemical parameters: The biochemical parameters were determined on day 12 after the animals were sacrificed by cervical dislocation. Total cholesterol, triglycerides (TGL), highdensity lipoprotein (HDL) and lowdensity lipoprotein (LDL), were determined by the glucose oxidase method, using an autoanalyzer[14,15].
Statistical analysis: Results of estimation of biochemical and functional parameters have been reported as mean value ±SEM. The variation in a set of data has been estimated by performing one way analysis of variance (ANOVA). Individual comparisons of group mean values were done using Dunnet’s test (Sigma stat 3.5). P values <0.05, were considered statistically significant. |
Back to Top  |
|
 |
 |
|
The result of acute toxicity study of methanol extracts of Ficusrecemosaon laboratory animals showed that the animals were safe up to a maximum dose of 2000 mg/kg body weight. The effects of 50% and 70% methanol extract of leaves of Ficusrecemosa, on the plasma glucose level are illustrated in table 1. Both extracts at dose of 500 mg/kg body weight showed significant reduction in plasma glucose level in rats at 30 min, and same was observed in standard drug. At 90 min, 250 mg/kg and 500 mg/kg body weight of both extracts treated rats produces significant reduction in plasma glucose level, while in glucose control rats, plasma glucose level was increased.
The imitation of diabetes in the experimental rats was confirmed by the presence of a high fasting plasma glucose level. The effect of 50% and 70% methanol extract at different doses of Ficusrecemosa, on fasting plasma glucose level of normal and streptozotocin induced are depicted in table 2. The difference between the experimental and control rats in lowering the fasting plasma glucose levels were statistically significant in diabetic rats.
Table 1: Effect of different extracts of Ficusrecemosaon blood glucose level in glucose primed rats
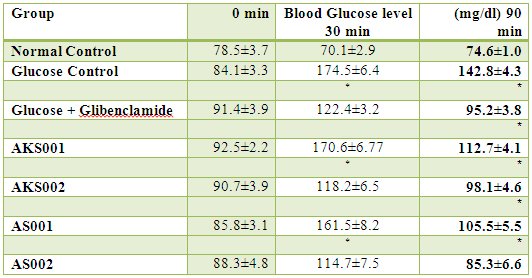
Values are expressed as mean ± SEM (Number of animals, n=6); significantly different at
*
P<0.05, when compared with glucose control group.
The effect of different doses of the both extracts on diabetes induced hyperlipidemia was also evaluated. It was observed that due to diabetes there was an increase in the total cholesterol levels as well as triglyceride levels. The HDL levels were reduced in the diabetic animals and the LDL levels were increased significantly (Table 3).
The 50% and 70% methanol extract showed a significant decrease in the total cholesterol levels and triglyceride levels. It also increased the HDL level and was successful in suppressing the LDL levels as compared to the standard drug by both extracts (Table 3).

|
Back to Top  |
|
 |
 |
|
The result of acute toxicity study of methanol extracts of Ficusrecemosaon laboratory animals showed that the animals were safe up to a maximum dose of 2000 mg/kg body weight. There were no changes in normal behavior pattern and no signs and symptoms of toxicity and mortality were observed as per OECD guidelines both the extracts fall under class four values LD50 value being 2000 mg/kg. The pharmacological evaluations were therefore carried out at doses of 100 and 200mg/kg body weight.
The fundamental mechanism underlying hyperglycemia in diabetes mellitus involves overproduction and decreased utilization of glucose by the tissues. In our study, the difference observed between the initial and final fasting plasma glucose levels of different groups under investigation revealed a significant elevation in blood glucose in diabetic control group as compared with normal animals at the end of the 12day experimental period. When 50% and 70% methanol extract of leaves of Ficusrecemosawere administered to glucose loaded normal rats fasted for 18 h, decrease in plasma glucose level was observed after 30 min. Both the extracts reduced plasma glucose level to normal at 90 min. During study it was found that both extracts control significantly the blood glucose level on streptozotocin induced diabetic rats. The methanol extracts of leaves induced a significant reduction on blood glucose level in STZinduceddiabetic rats as compared to the diabetic control group. But 70% methanol extract of leaves showed more significant antidiabetic activity as compared to 50% methanol extract. The possible mechanism by which Ficusrecemosabrings about its hypoglycemic action in diabetic rat may be by potentiating the insulin effect of plasma by increasing either the pancreatic secretion of insulin from the existing beta cells or by its release from the bound form.
The marked increase in serum triglycerides and cholesterol observed in untreated diabetic rats. Under normal circumstances insulin activates enzyme lipoprotein lipase and hydrolyses triglycerides. Insulin deficiency results in failure to activate the enzymes thereby causing hypertriglyceridemia. The significant control of the levels of serum lipids in the both extracts of leaves treated diabetic rats may be directly attributed to improvements in insulin levels upon Ficusrecemosatherapy. Elevation of plasma lipid concentration in diabetes is well documented. In insulin deficient diabetics, the plasma free fatty acid concentration is elevated as a result of increased free fatty acid outflow from fat depots, where the balance of the free fatty acid esterification–triglyceride lipolysis cycle is displaced in favour of lipolysis. Induction of diabetes with STZ is associated with the characteristic loss of body weight which is due to increased muscle wasting in diabetes. Diabetic rats treated with the extracts showed an increase in body weight as compared to the diabetic control which may be due to its protective effect in controlling muscle wasting i.e. reversal of gluconeogenesis[16,17].
Abnormalities in lipoproteins are very common in both NIDDM and IDDM. Although lipoprotein alterations appear to be an intrinsic part of these disorders, such alterations are also induced by diabetes associated complications such as obesity and renal disease. The total cholesterol, triglyceride levels, LDL and LDL were observed to be elevated in diabetics but reduced by both extracts as well as glibenclamide showing their beneficial effects. In the present study, HDL levels remained unchanged in diabetics compared to the other groups. These results suggest the beneficial effects of the natural extract in improving the
imbalance in lipoprotein metabolism are also comparable to those of glibenclamide.
The 70% methanol extract of leaves produced more significant antidiabetic activity than 50% methanol extract, this indicates that the phytoconstituents responsible for antidiabetic activity are more soluble in 70% methanol. The present study has indicated the fact that the plant Ficusrecemosa, has antidiabetic and antihyperlipidemic constituents and production of a safe antidiabetic drug is very much possible from leaves.
|
Back to Top  |
|
 |
 |
|
1. Pari L, Satheesh MA. Antidiabetic activity of BoerhaaviadiffusaL.: effect on hepatic key enzymes in experimental diabetes. Journal of Ethnopharmacology 2004; 91: 109 113.
2. Sumana G, Suryawashi SA. Effect of Vincaroseaextracts in treatment of alloxan diabetes in male albino rats. Indian Journal of Experimental biology 2001; 39: 748
3. Chakrabarti S, Biswas TK, Seal T, Rokeya B, Ali L, Azad Khan AK, Nahar N, Mosihuzzaman M, Mukherjee B. Antidiabetic activity of CaesalpiniabonducellaF. in chronic type 2 diabetic model in LongEvans rats and evaluation of insulin secretagogue property of its fractions on isolated islets. Journal of Ethnopharmacology 2005; 97: 117122.
4. Kannur DM, Hukkeri VI, Akki KS. Antidiabetic activity of Caesalpinia bonducella seed extracts in rat.s Fitoterapia 2006; 77: 546549.
5. Trivedi C, Shinde S, Sharma RC. Preliminary phytochemical and pharmacological studies on Ficusracemosaextract (Gular). Indian Journal of Medical Research 1969; 57(6): 10701074.
6. Mandal SC, Saha BP, Pal M. Studies on antibacterial activity of Ficusracemosaextract Linn. Phytotherapy Research 2000; 14: 278–280.
7. Khan N, Sultana S. Chemomodulatory effect of Ficusracemosaextract against chemically induced renal carcinogenesis and oxidative damage response in Wistar rats. Life Sciences 2005; 77: 11941210.
8. Rao CV, Verma AR, Vijayakumar M, Rastogi S. Gastroprotective effect of standardized extract of Ficusglomeratafruit on experimental gastric ulcers in rats. Journal of Ethnopharmacology 2008; 115: 323326.
9. Ecobichon DJ. Fixed dose procedure guidline 420. The basis of toxicity testing. 2ed. New York, CRC Press; 1997.
10. Ghosh MN.In: Schild HO editor. Fundamentals of Experimental Pharmacology. Calcutta: Scientific Book Agency, 1984.
11. Shirwaikar A, Rajendran K, Punitha IS. Antidiabetic activity of alcoholic stem extract of Cosciniumfenestratumin streptozotocinnicotinamide induced type 2 diabetic rats. J Ethnopharmacol 2005; 97: 369374.
12. SinghJ, Sahu RK, Prasad DN, Jangde R, Gupta R. Evaluation of antidiabetic potential of Ougeiniaoojeinensisleaves in streptozotocininduceddiabetic rats. Pharmacologyonline 2011; 2: 10461052.
13. MasielloP, Broca C, Gross R, Roye M, Manteghetti M, HillaireBuys D, etal.Experimental NIDDM: Development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes 1998; 47: 224229.
14. Nyarko AK, Sittie AA, Addy ME. The basis for the antihyperglycaemic activity of Indigoferaarrectain the rat. Phytother Res 1993; 7: 14.
15. Barham D, Trinder P.An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst 1972; 97: 142145. |
Back to Top  |
|
 |
|








