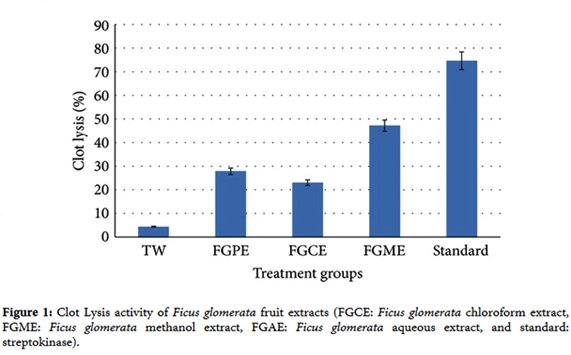
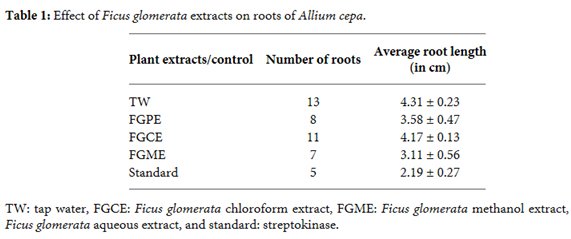
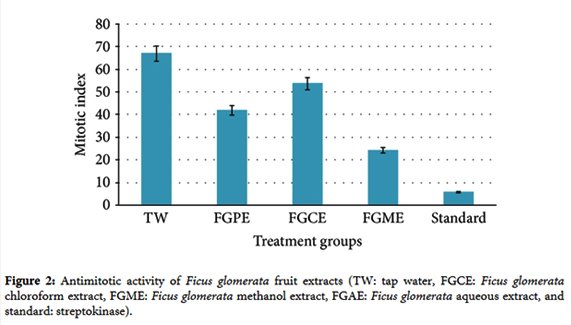
1. P. H. Kulkarni and A. Shahida, The Ayurvedic Plants, Satguru Publications, Delhi, India, 4th edition, 2004.
2. K. R. Kirthikar and B. D. Basu, Indian Medicinal Plants, International Book Distributors, Dehradun, India, 2nd edition, 1987.
3. I. J. Condit, Fig Varieties: A Monograph. Hilgardia, University of California, 1st edition, 1955.
4. S. C. Mandal, B. P. Saha, and M. Pal, “Study on antibacterial activity of Ficus racemosa Linn Leaf extract,” Phytotherapy Research, vol. 14, pp. 278–280, 2000.
5. F. Ahmed and A. Urooj, “Antioxidant activities, of various extracts of Ficus racemosa stem bark,” National Journal of Life Sciences, vol. 6, no. 1, pp. 69–74, 2009.
6. H. Wagnor, H. Hikino, R. Farnsworth, and N. R. London, “The economic significance of plants and their constituents as drug,” in Economic and Medicinal Plant Research, vol. 3, pp. 1–17, Academic Press, 1989.
7. J. Yamamoto, K. Yamada, A. Naemura, T. Yamashita, and R. Arai, “Testing various herbs for antithrombotic effect,” Nutrition, vol. 21, no. 5, pp. 580–587, 2005.
8. S. Dwivedi, “Terminalia arjuna Wight & Arn.-A useful drug for cardiovascular disorders,” Journal of Ethnopharmacology, vol. 114, no. 2, pp. 114–129, 2007.
9. D. Collen, “Coronary thrombolysis: streptokinase or recombinant tissue-type plasminogen activator?” Annals of Internal Medicine, vol. 112, no. 7, pp. 529–538, 1990.
10. A. Mannan, J. M. Kawser, A. M. A. Ahmed et al., “Assessment of antibacterial, thrombolytic and cytotoxic potential of Cassia alata seed oil,” Journal of Applied Pharmaceutical Science, vol. 1, no. 9, pp. 56–59, 2011.
11. B. Furie and B. C. Furie, “Mechanisms of thrombus formation,” The New England Journal of Medicine, vol. 359, no. 9, pp. 938–949, 2008.
12. J. C. Mucklow, “Thrombolytic treatment. Streptokinase is more economical than alteplase,” British Medical Journal, vol. 311, no. 7018, p. 1506, 1995. V. Rajkumar, G. Guha, R. Ashok Kumar, and L. Mathew, “Evaluation of cytotoxic potential of Acorus calamus rhizome,” Ethnobotanical Leaflets, vol. 13, pp. 832–839, 2009.
13. S. Auti, R. Pagare, D. Ahire, and V. Sawale, “Cytogenetical studies on the effect of omnacortil on root tip cells of Allium cepa L.,” Journal of Cell and Tissue Research, vol. 10, no. 3, pp. 2331–2335, 2010.
14. J. Angayarkanni, K. M. Ramkumar, T. Poornima, and U. Priyadarshini, “Cytotoxic activity of Amorphophallus paeoniifolius Tuber extracts in vitro,” American-Eurasian Journal of Agricultural and Environmental Sciences, vol. 2, no. 4, pp. 395–398, 2007.
15. M. A. Jordan, “Mechanism of action of antitumor drugs that interact with microtubules and tubulin,” Current Medicinal Chemistry. Anti-Cancer Agents, vol. 2, no. 1, pp. 1–17, 2002.
16. P. N. Saxena, L. K. S. Chauhan, and S. K. Gupta, “Cytogenetic effects of commercial formulation of cypermethrin in root meristem cells of Allium sativum: spectroscopic basis of chromosome damage,” Toxicology, vol. 216, no. 2-3, pp. 244–252, 2005.
17. S. Prasad, R. S. Kashyap, J. Y. Deopujari, H. J. Purohit, G. M. Taori, and H. F. Daginawala, “Development of an in vitro model to study clot lysis activity of thrombolytic drugs,” Thrombosis Journal, vol. 4, article 14, 2006.
18. G. Fiskesjo, “Allium test I: a 2-3 day plant test for toxicity assessment by measuring the mean root growth of onions (Allium cepa L.),” Environmental Toxicology and Water Quality, vol. 8, no. 4, pp. 461–470, 1993.
19. S. Bhattacharya and P. K. Haldar, “Evaluation of in vitro cytotoxic effect of Trichosanthes dioica root,” Pharmacognosy Research, vol. 2, no. 6, pp. 355–358, 2010.
20. G. Fiskesjö, “The Allium test - an alternative in environmental studies: the relative toxicity of metal ions,” Mutation Research, vol. 197, no. 2, pp. 243–260, 1988.
21. G. O. Williams and L. E. Omoh, “Mitotic effects of the aqueous leaf extract of Cymbopogon citratus in Allium cepa root tips,” Cytobios, vol. 1996, no. 350, pp. 161–168, 1996.
22. W. F. Grant, “Chromosome aberration assays in allium. A report of the U.S. Environmental Protection Agency Gene-Tox Program,” Mutation Research, vol. 99, no. 3, pp. 273–291, 1982.
23. A. Levan, “The effect of colchicine on root mitosis in Allium cepa,” Hereditas, vol. 24, pp. 471–486, 1938.
24. S. Prasad, R. S. Kashyap, J. Y. Deopujari, H. J. Purohit, G. M. Taori, and H. F. Daginawala, “Effect of Fagonia Arabica (Dhamasa) on in vitro thrombolysis,” BMC Complementary and Alternative Medicine, vol. 7, article 36, 2007.
25. W. M. Gesler, “Therapeutic landscapes: medical issues in light of the new cultural geography,” Social Science and Medicine, vol. 34, no. 7, pp. 735–746, 1992.
|