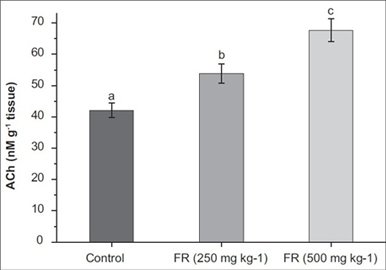
|
1. Dhingra D, Parle M, Kulkarni SK. Memory enhancing activity of Glycyrrhiza glabra in mice. J Ethnopharmacol. 2004;91:361–5. [PubMed]
2. Uriarte PI, Calvo MI. Phytochemical study and evaluation of antioxidant, neuroprotective and acetylcholinesterase inhibitor activities of Galeopsis ladanum L. extracts. Pharmacogn Mag. 2009;5:287–90.
3. Nabeshima T. Behavioral aspects of cholinergic transmission: Role of basal forebrain cholinergic system in learning and memory. Prog Brain Res. 1993;98:405–11. [PubMed]
4. Ringman JM, Cummings JL. Metrifonate: Update on a new antidementia agent. J Clin Psychiatry.1999;60:776–82. [PubMed]
5. Sramek JJ, Frackiewicz EJ, Cutler NR. Review of acetylcholinesterase inhibitor galanthamine. Expert Opin Investig Drugs. 2000;9:2393–402. [PubMed]
6. Mashkovskii MD, Glushkov RG. Drugs for the treatment of Alzheimer's disease. Pharm Chem J.2001;35:179–82.
7. Potkin SG, Anand R, Fleming K, Alva G, Keator D, Carreon D, et al. Brain metabolic and clinical effects of rivastigmine in Alzheimer's disease. Int J Neuropsychopharmacol. 2001;4:223–30. [PubMed]
8. Balaraman R, Shingala J. Nootropic. Indian J Pharmacol. 2002;34:439–40.
9. Sugimoto H, Ogura H, Arai Y, Iimura Y, Yamanishi Y. Research and development of donepezil hydrochloride, a new type of anticholinesterase inhibitor. Jpn J Pharmacol. 2002;89:7–20. [PubMed]
10. Gauthier S, Emre M, Farlow MR, Bullock R, Grossberg GT, Potkin SG. Strategies for continued successful treatment of Alzheimer's disease: Switching cholinesterase inhibitors. Curr Med Res Opin.2003;19:707–14. [PubMed]
11. Bores GM, Huger FP, Petko W, Mutlib AE, Camacho F, Rush DK, et al. Pharmacological evaluation of novel Alzheimer's disease therapeutics: Acetylcholinesterase inhibitors related to galanthamine. J Pharmacol Exp Ther. 1996;277:728–38. [PubMed]
12. Kartal M, Orhan I, Abu-Asaker M, Senol FS, Atici T, Sener B. Antioxidant and anticholinesterase assets and liquid chromatography-mass spectrometry preface of various fresh-water and marine macroalgae. Pharmacogn Mag. 2009;5:291–7.
13. Orhan I, Sener B, Choudhary MI, Khalid A. Acetylcholinesterase and butrylcholinesterase inhibitory activity of some Turkish medicinal plants. J Ethnopharmacol. 2004;91:57–60. [PubMed]
14. Ortega MG, Agnese AM, Cabrera JL. Anti-cholinesterase activity in an alkaloid extracts of Huperzia saururus. Phytomedicine. 2004;11:539–43. [PubMed]
15. Viegas C, Bolzani VS, Pimentel LS, Castro NG, Cabral RF, Costa RS, et al. New selective acetylcholinesterase inhibitors designed from natural piperidine alkaloids. Bioorg Med Chem.2005;13:4184–90. [PubMed]
16. Roodenrys S, Booth D, Bulzomi S, Phipps A, Micallef C, Smoker J. Chronic effects of Brahmi (Bacopa monnieri) on human memory. Neuropsychopharmacology. 2002;27:279–81. [PubMed]
17. Ahmed F, Urooj A. Anticholinesterase activities of cold and hot aqueous extracts of F. racemosastem bark. Pharmacogn Mag. 2010;6:140–2. [PMC free article] [PubMed]
18. McGeer EG, McGeer PL. Brain inflammation and the therapeutic implications. Curr Pharm Des.1999;5:821–36. [PubMed]
19. Ebrahimzadeh MA, Ehsanifar S, Eslami B. Sambucus ebulus elburensis fruits: A good source for antioxidants. Pharmacogn Mag. 2009;5:213–8.
20. Li RW, Leach DN, Myers SP, Lin GD, Leach GJ, Waterman PG. A new anti-inflammatory glucoside from Ficus racemosa L. Planta Med. 2004;70:421–6. [PubMed]
21. Ahmed F, Urooj A. Antioxidant activity of various extracts of Ficus racemosa stem bark. Nat J Life Sci. 2009;6:69–74.
22. Sigurdsson EM, Hejna MJ, Lee JM, Lorsen SA. Beta-Amyloid 25-35 and/or quinolinic acid injections into the basal forebrain of young male Fischer-344 rats: Behavioral, neurochemical and histological effects. Behav Brain Res. 1995;72:141–56. [PubMed]
23. O’Neill JJ, Sakamoto T. Enzymatic fluorometric determination of acetylcholine in biological extracts.J Neurochem. 1970;17:1451–60. [PubMed]
24. Parle M, Singh N. Animal models for testing memory. Asia Pac J Pharmacol. 2004;16:101–20.
25. Winter SC, Szabo-Aczel S, Curry CJ, Hutchinson HT, Hogue R, Shug A. Plasma carnitine deficiency.Clinical observation in 51 pediatric patients. Am J Dis Child. 1987;141:660–5. [PubMed]
26. Sato A, Sato Y, Uchida S. Regulation of cerebral cortical blood flow by basal forebrain cholinergic fibers and aging. Auton Neurosci. 2002;96:13–9. [PubMed]
27. Geula C, Mesulam M. New York: Raven Press; 1994. Cholinergic systems and related neuropathological predilection patterns in Alzheimer disease; pp. 263–91.
28. Sivaraman D, Muralidaran P. Acetylcholine enhancing activity of methanol leaf extract of Ficus hispida Linn .in rat hippocampus. J Herb Med Toxicol. 2009;3:147–50.
29. Vyawahare NS, Bodhankar SL. Effect of Argyreia speciosa extract on learning and memory paradigms in mice. Pharmacogn Mag. 2009;5:43–8.
30. Sayre LM, Zagorski MG, Surewicz WK, Krafft GA, Perry G. Mechanisms of neurotoxicity associated with amyloid beta deposition and the role of free radicals in the pathogenesis of Alzheimer's disease: A critical appraisal. Chem Res Toxicol. 1997;336:1216–22. [PubMed]
31. Ahmed F, Urooj A. Glucose lowering, hepatoprotective and hypolipidemic activity of stem bark ofFicus racemosa in streptozotocin-induced diabetic rats. J Young Pharm. 2009;1:160–4.
32. Balas RK, Agha R. Isolation of a hypoglycemic principle from the bark of Ficus glomerata Roxb.Chem Pharm Bull. 1985;2:13–4.
33. Joy PP, Thomas J, Mathew S, Skaria BP. Vol. 2. Calcutta: Naya Prokash; 2001. Medicinal Plants. Tropical Horticulture; p. 449. 632.
34. Khan N, Sultana S. Chemomodulatory effect of Ficus racemosa extract against chemically induced renal carcinogenesis and oxidative damage response in Wistar rats. Life Sci. 2005;29:1194–210.[PubMed]
35. Malairajan P, Gopalakrishnan G, Narasimhan S, Veni JK. Analgesic activity of some Indian medicinal plants. J Ethnopharmacol. 2006;106:425–8. [PubMed]
36. Nguyen TD, Pham DT, Nguyen TD, Chau VM. Some compounds isolated from Momordica cochinchinensis seed and Ficus glomerata bark. Ly Va Sinh Hoc. 2001;6:66–9.
37. Rahman NN, Khan M, Hasan R. Bioactive components from Ficus glomerata. Pure Appl Chem.1994;66:2287–90.
38. Rahuman AA, Venkatesan P, Geetha K, Gopalakrishnan G, Bagavan A, Kamaraj C. Mosquito larvicidal activity of gluanol acetate, a tetracyclic triterpenes derived from Ficus racemosa Linn. Parasitol Res. 2008;103:333–9. [PubMed]
39. Singhal RK, Saharia HS. Chemical examination of Ficus glomerata Roxb. Herba Hungarica.1980;19:17–20.
40. Orhan I, Sener B. Acetylcholinesterase inhibitors from natural resources. FABAD J Pharm Sci.2008;28:51–8.
|