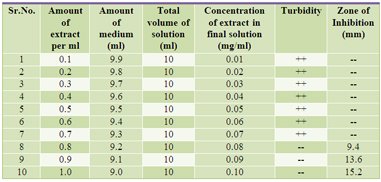
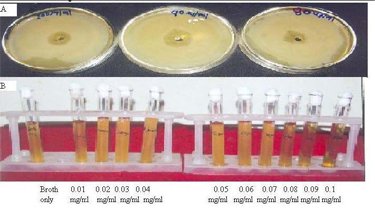
| [1] The Ayurvedic Pharmacopoeia of India,
Government of India, Ministry of
Health and Family Welfare, Department
of Indian System of Medicine and
Homeopathy, New Delhi.Ist Edition,
Part I, Vol. I, 117-118, (1990)
[2] Dr.K.M.Nadkarni’s,revised by
Dr.A.K.Nadkarni, Indian Materia
Medica,Bombay Popular Prakashan,
Mumbai, IInd reprint IIIrd revised
edition, 548-550,(2000)
[3] C.P.Khare, Indian Medicinal Plants An
Illustrated Dictionary, Springer (India)
Private Limited, New Delhi, Ist Indian
reprint,266-267,(2007)
[4] The Wealth Of India, A Dictionary Of
Indian Raw Materials and Industrial
Products (Raw Materials) National
Institute Of Science
Communication,Council of Scientific
and Industrial Reseach, New Delhi, Ist
Supp.Set.Vol III, 130, (2002)
[5] The Wealth Of India, A Dictionary Of
Indian Raw Materials and Industrial
Products (Raw Materials) National
Institute Of Science Communication,Council of Scientific
and Industrial Reseach, New Delhi, Ist
Reprint.Set.Vol IV, 35, (2002)
[6] William J,Hausler Jr MS,Topley &
Wilson’s Microbiology µbial
infectionsActinomyses, Actinobacillosis
and releated diseases( Great Britan –
Arnold) Vol.3 9th ed. 777-787,(1998).
[7] R.Ananthanarayan,C K J
Panikar,Textbook of Microbiology,
Orient Longman Limited,Madras,6th
ed.370-373,(1992)
[8] J.P. Duguid, B.P. Marmion, R. H. A.
Swain, MACKIE & McCARTNEY
Medical Microbiology, Vol
1,microbial infections, 13 th ed.
Churchill Livingstone,304,(1980)
[9] Michael J. Pelczar,JR., E.S.C. Chan,
Noel. R. Krieg,
Microbiology,TataMcGraw-Hill
publishing, 5th ed. 274-275,(1997)
[10] B.J Wadher, G.L Bhoosreddy, ‘Manuals
of Diagnostic Microbiology’, Himalaya
Publishing House,IST edition, 62-
67,(1995).
[11] Barry A.L.,The antimicrobial
susceptibility test principles and
practices, Lea and Febiger,
Philadelphia,163-164,(1976) |